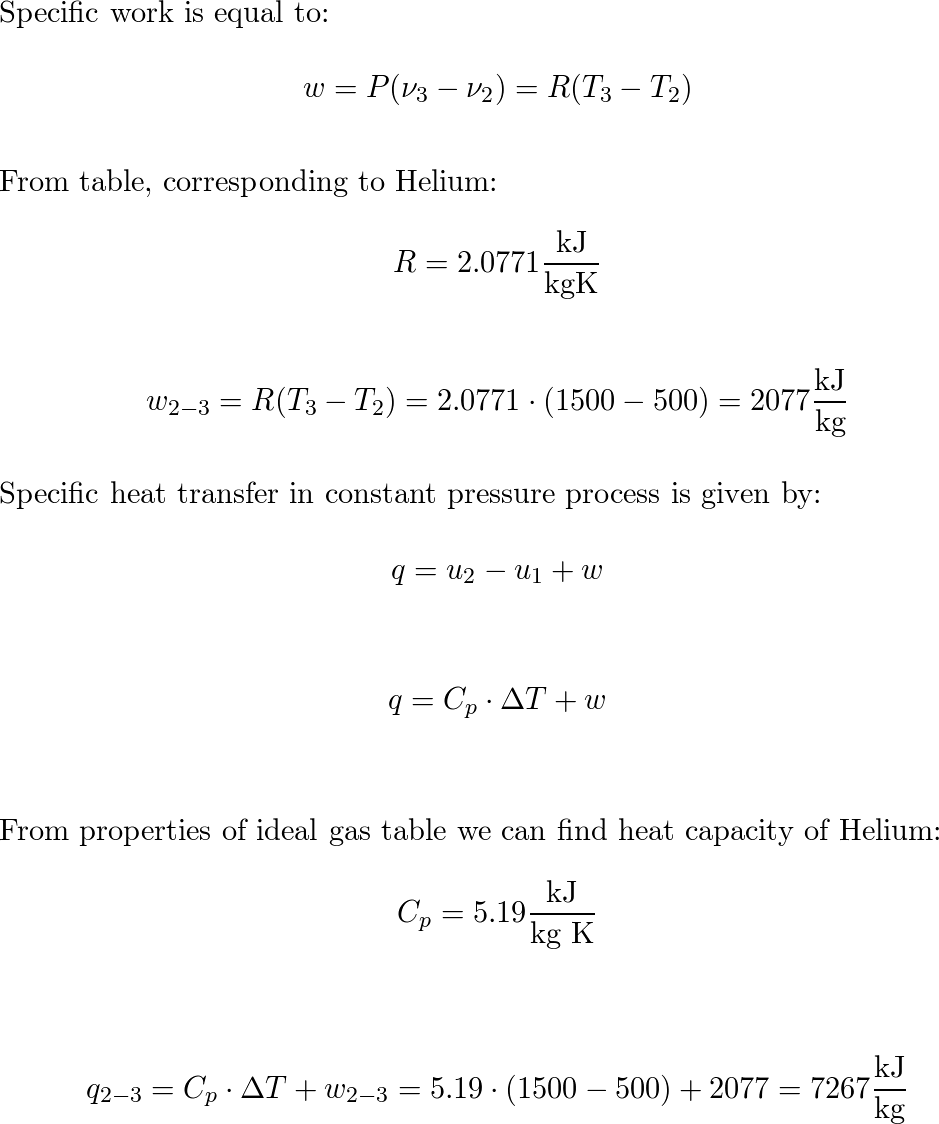
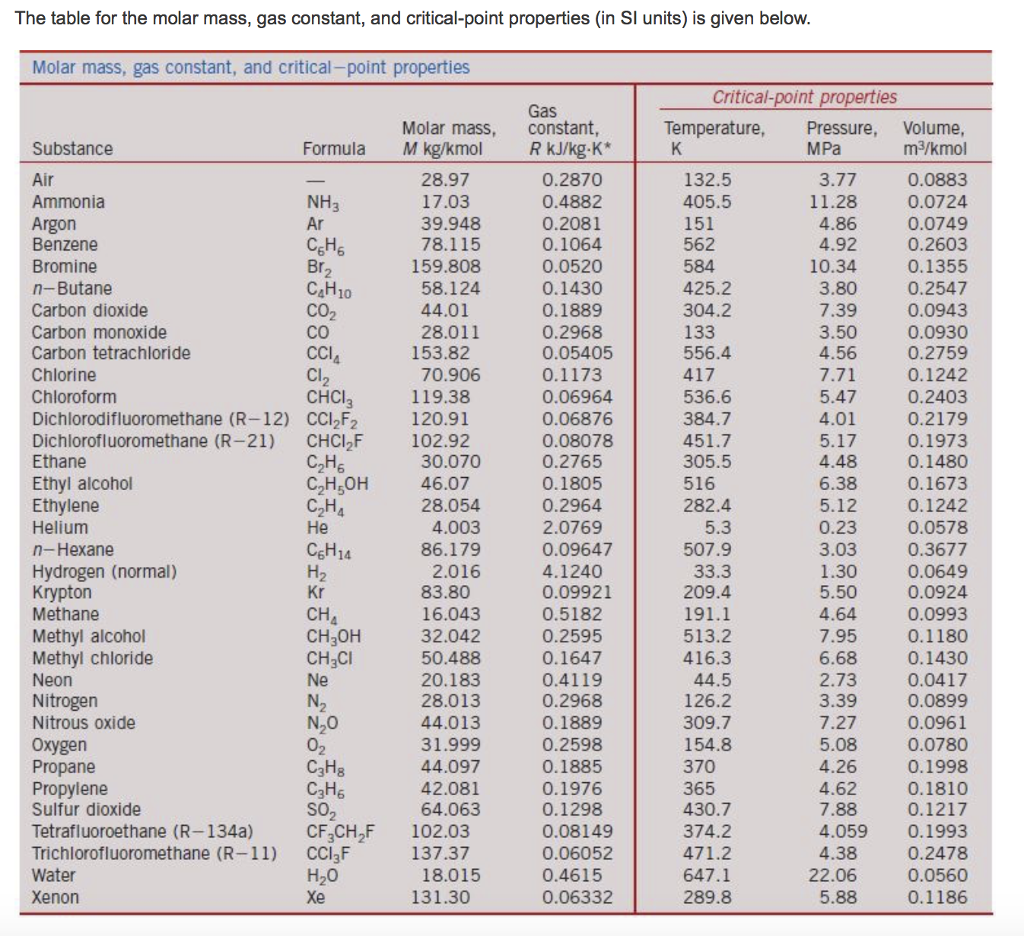
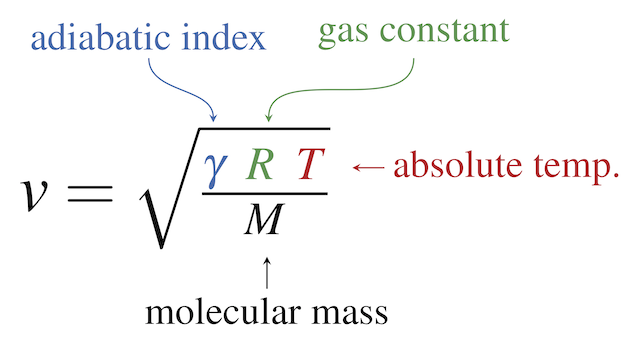
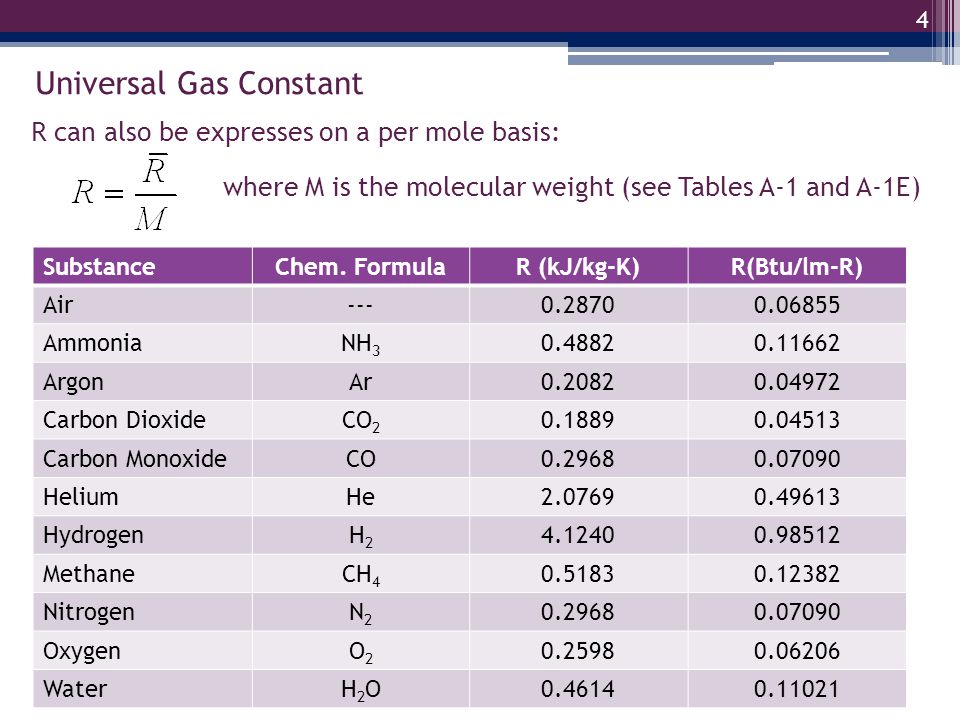
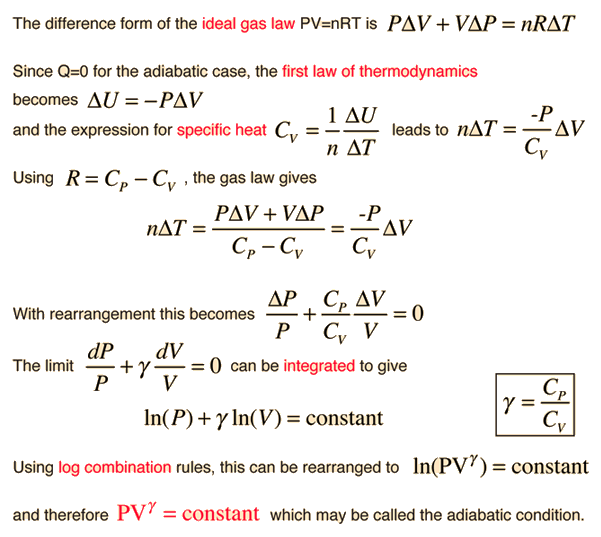
Half mole of Helium gas is contained in a container at STP. Heat energy needed to double pressure of a gas , keeping the volume constant(heat capacity of gas =3j/gm K^°) 1)3276j

A balloon is filled with 1 mole of helium gas at 100 kPa of pressure and a temperature of 300 K. What is - brainly.com
Helium (He) gas is heated from 200 K to 400 K at constant pressure, the entropy change for 1 mole of the gas will be how to solve this problem?

Hot Selling Helium Balloon Gas 99.999% Helium Gas Cheap Price - China Helium Gas Constant, Helium Gas Cost | Made-in-China.com
What is the pressure (atm) of 3.5 moles of helium at -50 °C in a rigid container whose volume is 25.0 L? | Socratic

In a thermodynamic process helium gas obeys the law TP2/5=constantt. The heat given 'n' moles of He in order to raise the temperature from T to 2T is.
Calculate the difference between between the principal specific heat capacity of 1g He at STP (R = 8.13 J/K Mol, J = 4.186 J /cal and molecular weight = 4 > - EduRev Class 11 Question

The picture (not to scale) shows a pV diagram for 3.6 g of a helium gas (He) that undergoes the process 1 to 2 to 3 to 1. The ideal gas constant
![Two moles of Helium gas are to be taken over the cycle \\[{\\text{ABCDA}}\\]as shown (below) in the \\[{\\text{P - T}}\\]diagram. (Assume the gas to be ideal and R is a gas constant.).Now, Two moles of Helium gas are to be taken over the cycle \\[{\\text{ABCDA}}\\]as shown (below) in the \\[{\\text{P - T}}\\]diagram. (Assume the gas to be ideal and R is a gas constant.).Now,](https://www.vedantu.com/question-sets/0cb3c7f1-24ca-4773-848c-92578a8aa4e55598971945812544228.png)
Two moles of Helium gas are to be taken over the cycle \\[{\\text{ABCDA}}\\]as shown (below) in the \\[{\\text{P - T}}\\]diagram. (Assume the gas to be ideal and R is a gas constant.).Now,

OneClass: The molecular mass of helium is 4 g/mol, the Boltzmann's constant is 1.38066 × 10-23 J/K, t...