If first ionisation energy of He atom is "x eV/atom" then energy required to remove both electrons of the He - Brainly.in

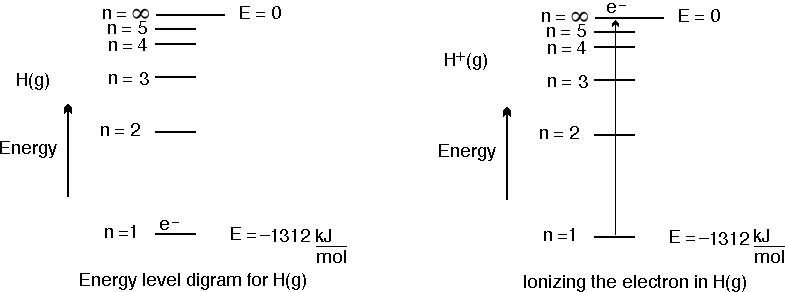
With the help of Bohr \'s model , calculate the second ionisation energy of helium (energy requi... - YouTube

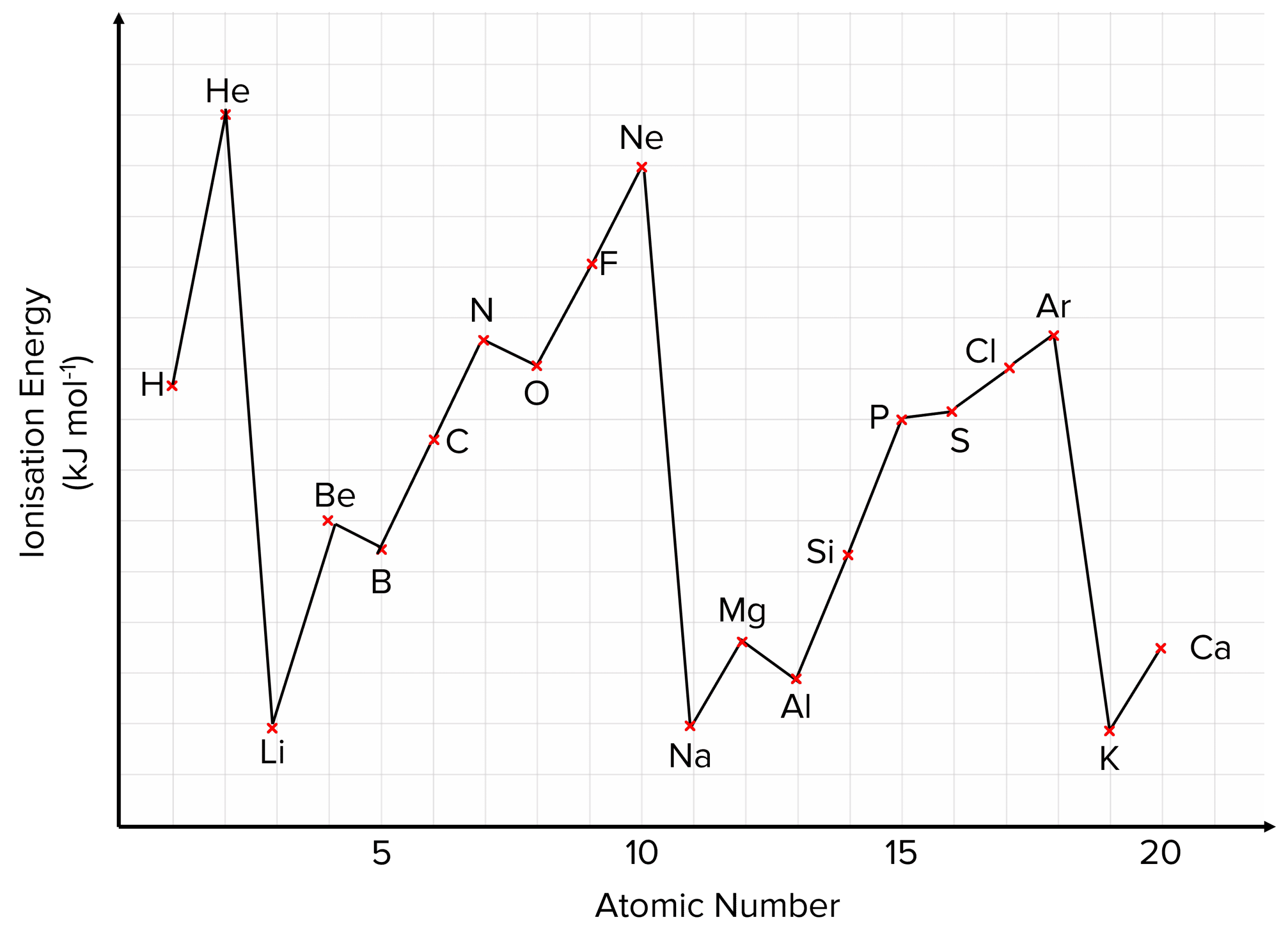
ATOMIC NUMBER 1st IONISATION ENERGY / kJmol -1 Variation in 1st Ionisation Energy EXPLANATION Despite having a nuclear charge of only 1+, Hydrogen has. - ppt download
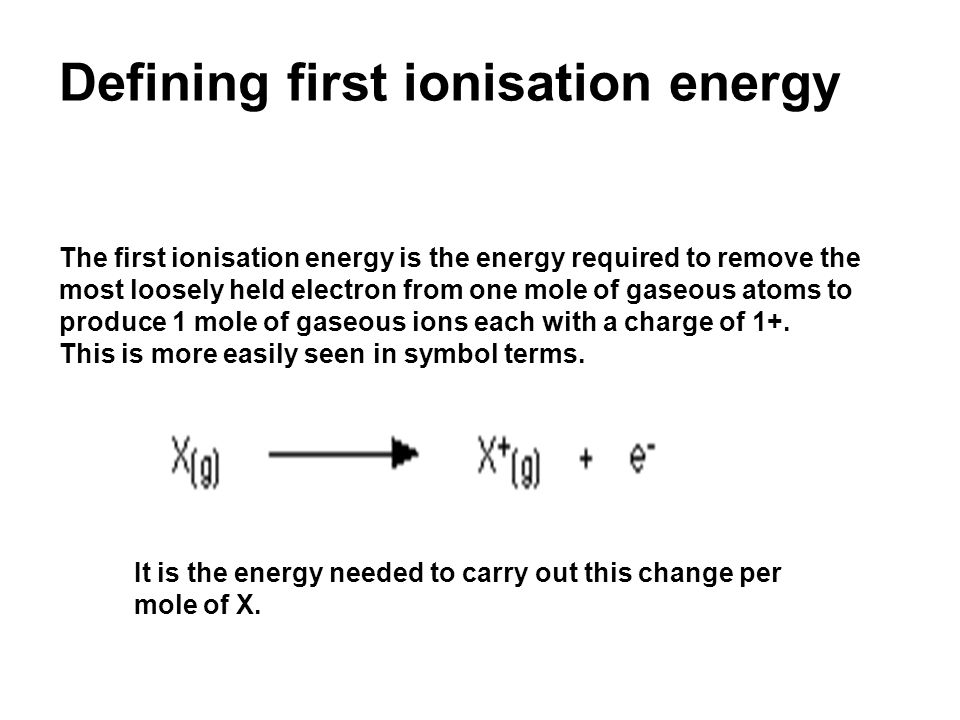
Defining first ionisation energy The first ionisation energy is the energy required to remove the most loosely held electron from one mole of gaseous atoms. - ppt download

The first and second ionization potentials of helium atoms are 24.58 eV and 54.4 eV respectively... - YouTube

The ionisation energy of hydrogen atom is 13.6 eV, the ionisation energy of helium atom would be (1988) (a) 13.6 eV (b) 27.2 eV (c) 6.8 eV (d) 54.4 eV

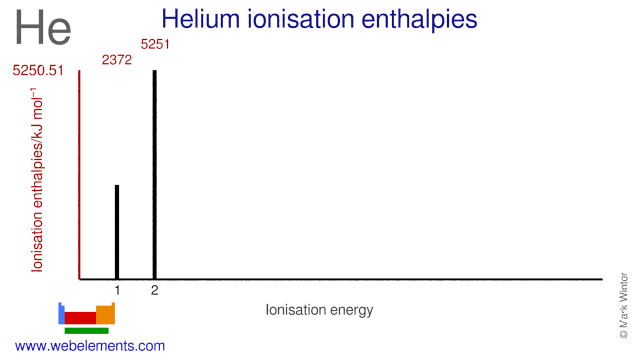
![Ionization energy [eV] of helium atom and molecular ions. | Download Table Ionization energy [eV] of helium atom and molecular ions. | Download Table](https://www.researchgate.net/publication/1790597/figure/tbl2/AS:667036099289106@1536045207928/Ionization-energy-eV-of-helium-atom-and-molecular-ions.png)